In Chemistry weight of some elements are measure in the form of Average Atomic Mass. Isotopes are found in different abundances in nature. Certain elements have many isotopes and certain elements have few isotopes. Regardless of the number of the natural isotopes, the weighted average mass takes into consideration not only the mass of each isotope, but what also its natural abundance in terms of percent as found in the nature. So when you have the mass of two isotopes of an element and are given its natural percent abundance then you can computer its average atomic weight. Similarly if you have the average atomic weight of an element and the percent of its natural abundance then you can find the mass of different isotopes of the element in a given matter. This video talks about isotopes of an element and its average atomic weight.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.




















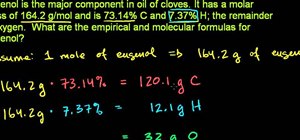




Be the First to Comment
Share Your Thoughts