C For Chemistry delves into the chemistry of science experiments. This chemist knows what he's talking about. These chemistry experiments are not only fun, but very educational for all of those interested in scientific chemical reactions and properties.
This video tutorial will show you how to determine enthalpy change in the hydration of MgSO4 (anhydrous magnesium sulfate).
CHEMISTRY OF IT:
Magnesium sulfate is used as an example to illustrate how enthalpy change is determined. Hydrated MgSO4 and anhydrous MgSO4 is dissolved separately and the temperature change is recorded.
DeltaH of anhydrous MgSO4 - DeltaH of hydrated MgSO4 = enthalpy of hydration.
Check out more of C for Chemistry science videos on WonderHowTo.
Music: Blue Scorpion by Kevin MacLeod @ Incompetech
Licensed under Creative Commons "Attribution 3.0"
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.




















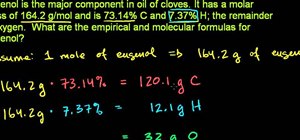



Be the First to Comment
Share Your Thoughts