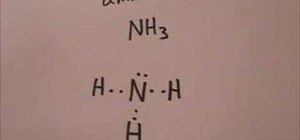
In this how-to video, you will learn how to make the Lewis structure for Ammonia. The formula for Ammonia is NH3. Now, write down H, N, and H in a horizontal line. Place an H under the N. Place two dots in between the spaces found in the H's and the N. Also place two dots above the N. Since the valance electrons are balanced, draw a line between the two dots connecting the H to the N. Leave the two dots above the N alone. This video shows just how easy it is to create a Lewis structure for Ammonia.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.























1 Comment
The concept of relative pKa values, as mentioned by others, is essential when discussing Lewis base strength. Comparing electron pairs alone does not provide sufficient information about the strength of a Lewis base. For instance, NH3 has a pKa value of approximately 35, indicating a strong tendency to donate its electrons to form a more stable conjugate acid. In contrast, water has a pKa value of around 15.7, which is orders of magnitude lower, suggesting a significantly weaker tendency to donate electrons.
Share Your Thoughts