This video is about Level 2 atomic concepts, specifically the Pennium Lab. This helps you understand how atomic masses are derived for the periodic table. This teacher demonstrates how to do the calculation. He first reviews the Isotopes as they are different masses of the same type of atoms. He uses chromium as his example. It has a different number of neutrons in the nucleus. Using the equation, it could have either 26, 28, 29 or 30, with its 24 protons. So he subtracts the atomic number from the atomic mass to get the number of neutrons. Universities typically have a machine called a Mass Spectrograph. You put a naturally occurring sample of the element into the machine and it sorts out the isotopes of the mass, reporting the AMU mass and the percentage of natural abundance for that mass. He derives the Fraction of Periodic Table Mass Number From This Isotope by multiplying the AMU Mass by the Percentage of Natural Abundance. Then, he adds up the Fractions of Periodic Table Mass Numbers to get the Periodic Table Mass.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.
















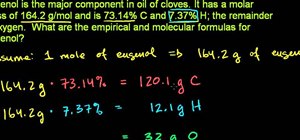







Be the First to Comment
Share Your Thoughts