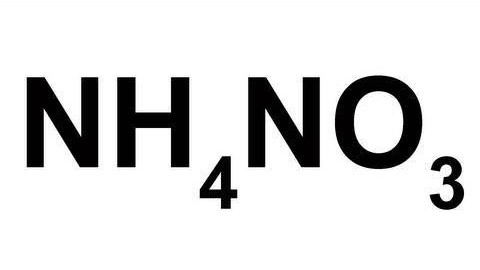
OK, so you don't necessarily have to make ammonium nitrate to have ammonium nitrate — ammonium nitrate can simply be obtained from fertilizers and instant cold packs. Making homemade NH4NO3 can be much more expensive then buying it, but this method is not meant to be a viable route, it's an exploration of science and chemistry.
From Nurd Rage, keep reading to learn how to make ammonium nitrate with sodium bisulfate (NaHSO4) and nitrate salt with Dr. Lithium.
WARNING: The chemicals are corrosive and ammonia has a horrible smell; work outside or in a fumehood and wear gloves.
- Get 138 grams of sodium bisulfate.
- Add just enough water to dissolve it, usually 300 mL.
- Get one mole equivalent of a pure nitrate salt. Some common salts include sodium nitrate(85 grams), potassium nitrate (101 grams), and calcium nitrate (118 grams (if using tetrahydrate)).
- Dissolve the nitrate in a minimum of water.
- Mix the two solutions together.
- Neutralize the mixture with ammonia (using a pH meter to determine the endpoint).
- Filter the mixture to get rid of any insoluble materials if needed.
- Then, boil until sodium sulfate begins to precipitate.
- Cool the mixture to 0ºC and filter.
- Dry the filtrate to obtain ammonium nitrate mixed with some leftover metal sulfates.
For higher purity, the solids can be mixed with 500mL of methanol, which selectively dissolves ammonium nitrate. Filter again and evaporate the filtrate to obtain pure ammonium nitrate.
Just updated your iPhone? You'll find new features for TV, Messages, News, and Shortcuts, as well as important bug fixes and security patches. Find out what's new and changed on your iPhone with the iOS 17.6 update.






















Be the First to Comment
Share Your Thoughts