How To: Build a Simple Paper Bridge as a Science Experiment
Every day we pass bridges, whether it's a foot bridge, a highway overpass, a span over water, or a viaduct over a valley. We pass on these structures without even thinking of the engineering genius that went into their design and construction, let alone the science behind their strength.

How To: Make This Amazing 9-Layer Density Tower from Things Found in Your Kitchen
Ralph Waldo Emerson once observed that "the seed of science" was "wonder," and taking a look at this nine-layer liquid tower from Steve Spangler's Sick Science! channel, one can't help but do just that — wonder. How is this possible? Is this magic or what?

How To: The Best Investigatory Projects in Science: 16 Fun & Easy Ideas to Kickstart Your Project
Most of us have conducted an investigatory science project without even knowing it, or at least without knowing that's what it was called. Most science experiments performed, from elementary to high school students and all the way up to professional scientists, are investigatory projects.

News: Vortex Cannon Demolishes House
Jem Stansfield from BBC's Bang Goes the Theory has "put scientific theory to the test" with his Vortex Cannon. Filmed at 1300-fps, you can see the cannon knock down three different houses made of straw, stick, and brick with an explosive vortex ring.

How To: Make Soap Out of Guava Leaf Extract for a Science Investigatory Project
Unless you're a high-schooler building a nuclear fusion reactor, the hardest part of a science investigatory project often is coming up with a good idea. You want it to be cool yet feasible, novel but still useful.

How To: Extract DNA from a Strawberry with Basic Kitchen Items
We all know that DNA is pretty amazing, but it's not something that most of us get much hands-on experience with. Even though it's in every living thing around us, we never see it, so we rarely think about it either.

News: What Happens When You Mix Coca Cola and Milk
Interesting reaction coke and milk The reaction of phosphoric acid (V) to proteins in the milk - they are cut and causes a precipitate

How To: Make a Paper Plate Speaker That Actually Works for Under $1
Back in 2007, YouTube user HouseholdHacker posted a parody video on how to make a high-def speaker for under a buck. MythBusters took on the challenge and busted it.

How To: Make a Monster Dry Ice Bubble
Sure it's been done before, but it never gets old. There's something magical about dry ice, bubbles, and especially the result you see when they're combined!

How To: Determine volume measurement
In this how to video you will learn how to measure the volume of solids and liquids. The formula for determining volume is width x length x height.

How To: Make fire 4 ways without matches by using chemistry
Watch this science video tutorial from Nurd Rage on how to make fire 4 ways without matches by using chemistry, without matches or lighters.

How To: DIY Ninja Turtle Ooze! Make Your Own Radioactive Canister of Glowing Green Slime at Home
There's a broken canister of mutant ooze leaking down into the sewers! But don't worry because this sticky slime is non-toxic, and it's so easy to make, a three-year-old can do it!
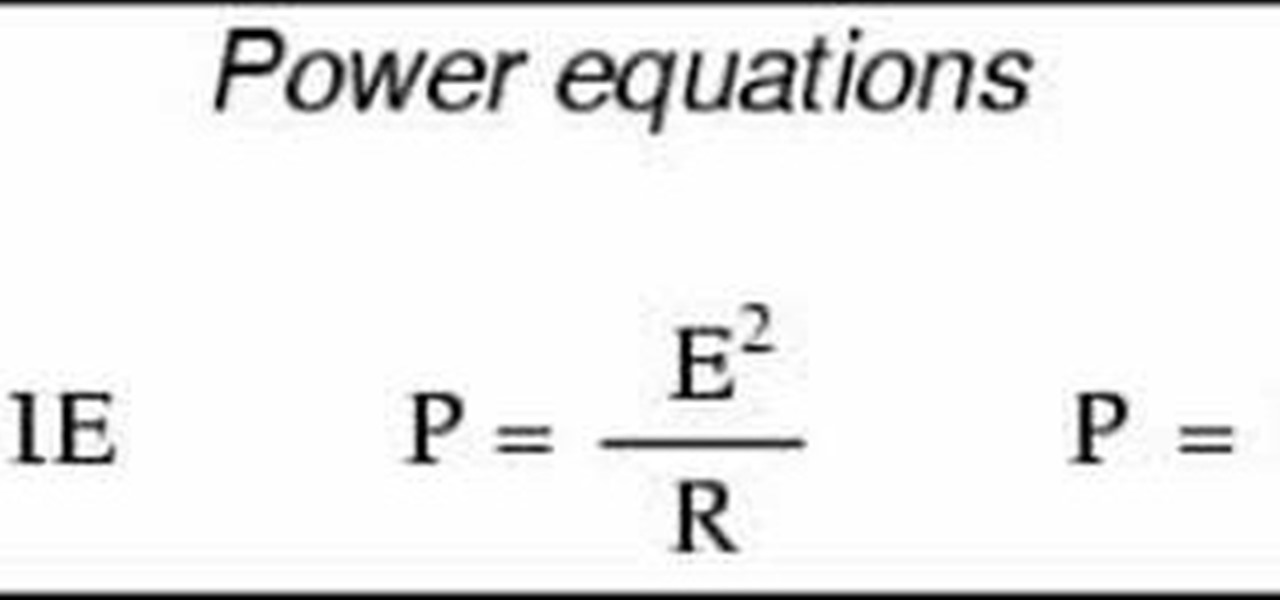
How To: Calculate electrical power
This video introduces the formulae for watts in an electric circuit, P=IxE, P=I^R, and P=E^/R. It also explains how P=I^R and P=E^/R are algebraically derived from P=IxE and Ohm's Law.
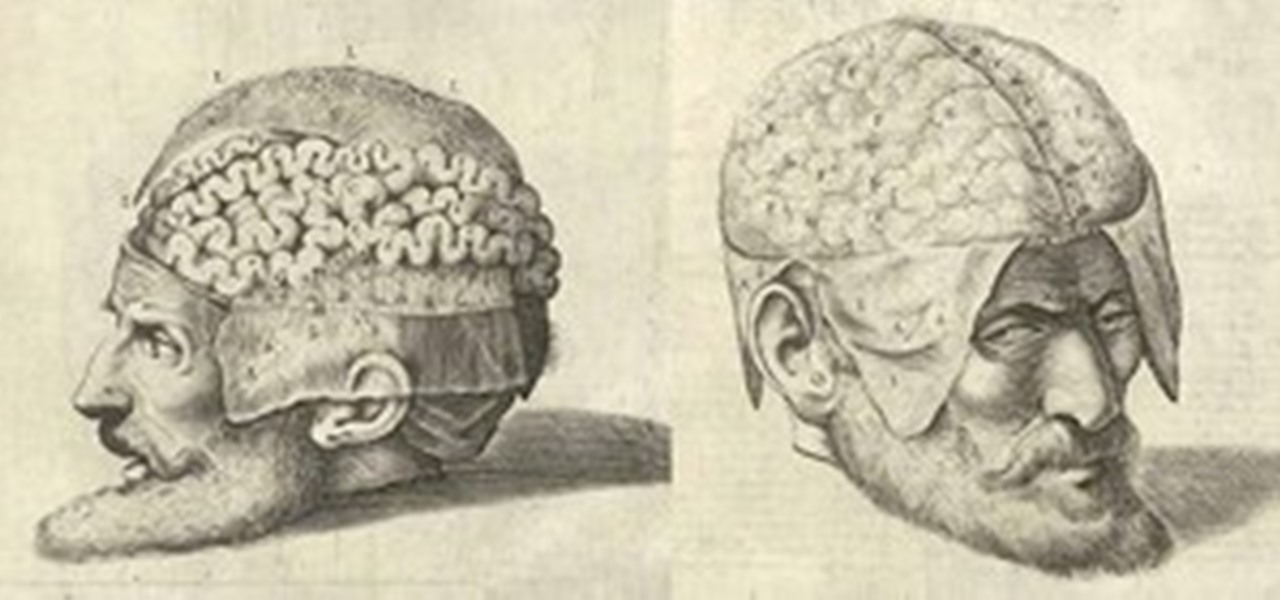
News: Dissecting a Human Head Through Anatomical Illustrations
Human anatomy is something every physician must undergo as a medical student. Some move on to become great doctors, some move on to become great artists, helping to better educate students and improve upon many illustrated representations of the human body since the days of medieval medicine. But thankfully, you don't have to be in the medical profession to enjoy the beautiful art of the human body created for teaching purposes.

How To: Make a Crazy Foam Explosion Science Experiment
Check out this video to see our Fantastic Foamy Fountain in action. The experiment uses Hydrogen peroxide and dry yeast. Hydrogen peroxide is similar to water but has an extra oxygen atom. This makes it more dangerous, and only adults should handle the hydrogen peroxide.

Classic Chemistry: Colorize Colorless Liquids with "Black" Magic, AKA the Iodine Clock Reaction
Want to make boring old colorless water brighten up on command? Well, you can control the color of water with this little magic trick. Actually, it's not really magic, but a classic science experiment known commonly as the iodine clock reaction, which uses the reactions between water and chemicals to instantly colorize water, seemingly by command. You can use different colorless chemicals to produce different colors, and you can even make the color vanish to make the water clear again.

Make Slime Without Borax: 5 Easy Recipes for Gooey Homemade Ooze
One of the only things I remember from watching Nickelodeon as a kid is the epic green slime. Looking back, I don't know what was so great about it, but every kid my age thought that being drenched in slime would be the coolest thing on earth.

How To: Make hydrochloric acid from salt
In this tutorial, we learn how to make hydrochloric acid from salt. First, you will pour some salt into a distil flask. After this, you will add in some concentrated sulfuric acid to the salt. Next, you will let these react with each other. You will start to see gasses bubble up and the excess hydrogen chloride gas come out through the top of the tube. To create a stronger reaction, you can add heat underneath the reaction. Then, test this by exposing it to ammonium chloride. If it's the righ...

How To: Make Water Freeze into Ice Instantaneously
Have you ever seen water freeze instantly? This "Quick Clip" shows some of my personal experiences with making instant ice using a bottle of water supercooled in a freezer.

How To: Make Your Own Homemade Glow Sticks
Glow sticks, a popular favor at parties and outdoor events, and a must-have on Halloween, can be traced back to the United States Navy in the mid-1960s. The military desired improved visibility during night operations, and glow sticks, with their small-size portability and lack of batteries, were a perfect tactical solution.

How To: Make lightning with a spoon and a balloon
In this video, we learn how to make lightning with a spoon and a balloon. First, you will need to gather a spoon and a balloon. Once you have these, blow up the balloon then tie it on the end so it's sealed. After this, rub the balloon on your hair and then slowly move the spoon towards the balloon. Turn the lights out and watch what happens. You will see sparks of electricity start to appear between the balloon and the spoon! This is great to do with children or as a quick experiment to show...

How To: There's Metal Hiding in Your Pepto-Bismol and Here's How You Extract It
Got an upset stomach or a little heartburn? America's favorite pink pill will cure it right up. But did you know that there's actually metal hiding in those chewable Pepto-Bismol tablets? Yes, metal. Technically, it's a poor metal, but metal's metal, right? Well, we do tend to eat a lot of iron in our diets, because it carries oxygen throughout our bodies, so consuming metallic minerals isn't anything abnormal. But you'd never think that Pepto-Bismol is actually made up of metal.

How To: Wrap your head around the concept of Entropy
Entropy can be a tricky concept to wrap your head around, but this clear and detailed video helps make it easy. By using a variety of props as examples, you too can master the idea of entropy to amaze and impress your friends!

How to Be Your Own SpaceX: Design, Build & Test Liquid-Fueled Rocket Engines
Move over NASA— SpaceX is taking over. Well, not really. But today, the privately funded spacecraft company broke all expectations when their Dragon capsule fell to a soft landing in the Pacific Ocean, completing an undoubtedly successful demo flight of nearly two full trips around Earth. It was the first re-entry of a commercial spacecraft ever, bringing commercial space transportation closer to reality.
How To: Make iodine easily
In this video, we learn how to make iodine easily. You will need potassium iodine and sulfuric acid to make this. First, add the acid into the potassium iodine slowly. After you add in each part, swirl the beaker slowly so it gets mixed together. After you have added in all of the potassium, you will place this into a beaker filled with ice water while you add in more, because the mixture gets really hot. When finished, you will end up with a mixture that is iodine and nothing else. Fill with...

How To: Make maganese heptoxide (permanganic acid)
This video tutorial is in the Education category which will show you how to make manganese heptoxide (permanganic acid). This procedure is extremely dangerous. Manganese heptoxide is an extremely powerful oxidizing agent. It has the ability to set fuels on fire from mere contact. Get an old dish and put a spatula full of potassium permanganate on it. Add a few drops of concentrated sulphuric acid. A green liquid is formed which is the manganese heptoxide. Now you can add any fuel like butanol...

News: World's Simplest Electric Train
The trick in the video is that the magnets are made of a conducting material and they connect the battery terminals to the copper wire, so the battery, magnets and copper wire make a circuit that generates a magnet field just in the vicinity of the battery. The geometry means the two magnets are automatically at the ends of the generated magnetic field, where the field is divergent, so a force is exerted on the magnets.

How To: Make (non-Newtonian) Oobleck from corn starch & water
Mr. O shows his audience in this video how to make oobleck, a slime-like substance which has a variety of unique properties. For this project, you will need a mixing bowl, food coloring, corn starch, a measuring cup, and water. First, color the water with food coloring to a color which is much darker than the color you would like. You will need the correct ratio of water to cornstarch, in a 1 to 2 ratio. Add some water to the bowl and add the cornstarch, then add the rest of the water. Finall...

How To: Make thunder in a test tube with ethanol & acetone
C For Chemistry delves into the chemistry of science experiments. This chemist knows what he's talking about. These chemistry experiments are not only fun, but very educational for all of those interested in scientific chemical reactions and properties.

How To: Build a vertical axis wind turbine
The economy is down, so what's one way you can save money? Build a vertical axis wind turbine! This eco-friendly four-part video tutorial will show you just how to make one so you can save money. These are detailed steps for making the vertical axis wind turbine. The blades can be easily interchanged offering different shapes and materials to experiment with. This design can create turbines up to 90 inches in diameter and up to 15 feet tall.

How To: Perform Separation by Decantation in the Chemistry Lab
Find out how everything in a chemistry lab works, from pipettes to burners to recrystallization to storage. You'll get precise instructions on how to work and perform certain scientific duties in the chem lab, whether it's chemical or just ordinary high school science.

How To: Use a thermometer to determine temperature
In this science tutorial learn how to measure temperature using a thermometer. This how to video goes over Celsius and Fahrenheit measurements.

How To: Determine the empirical and molecular formulas for a compound in chemistry
In this free video science lesson from Internet pedagogical superstar Salman Khan, you'll learn how to determine the empircal and molecular formulas of a substance given percent composition. Whether you need help studying for that next big test or could just use a hand finishing your homework, you're sure to be well served by this video lesson. For more information, including detailed, step-by-step instructions, take a look.
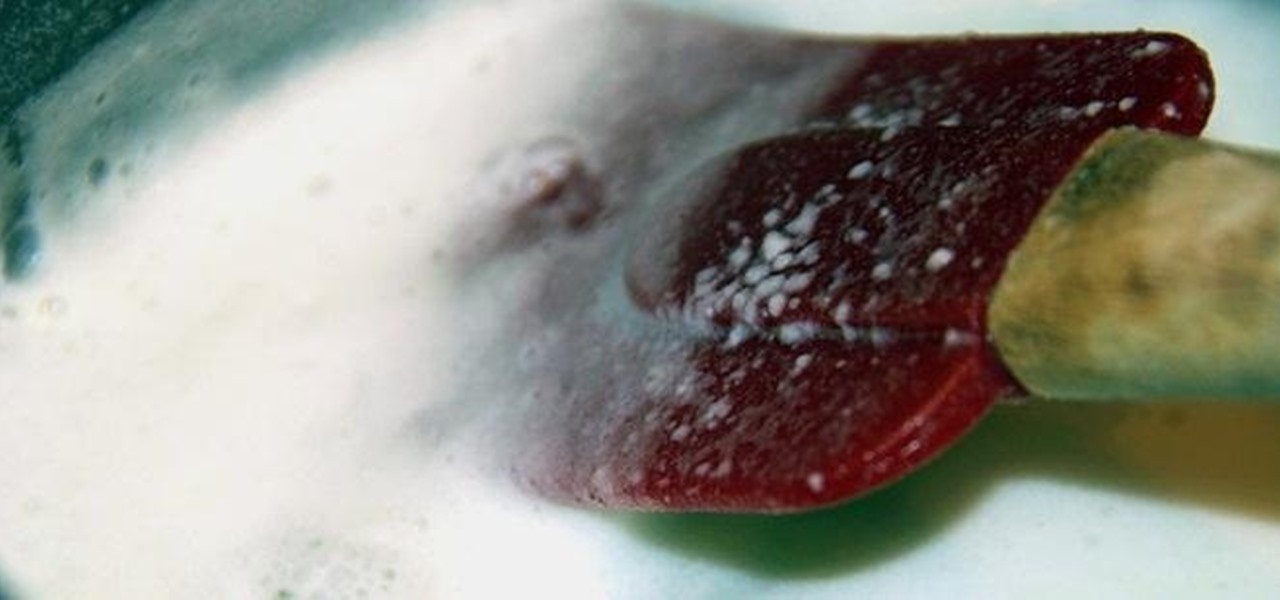
How To: Turn Milk into Strong Natural Glue with Baking Soda and Vinegar
You can do all kinds of unexpected things with milk, like make your own pore strips and invisible ink, or even get rid of red wine stains with it. But did you know that you can also use it to make your own glue?

How To: Make an Iodine Clock Reaction at Home
In this video, I'll be demonstrating how anyone can make their own iodine clock reaction with simple over-the-counter chemicals.

How To: Isolate the sugar in a can of soda
In this video from ScienceOnTheBrain we learn how to isolate the sugar in a can of soda. To find out how much sugar is in soda, pour a can into a pot and boil it until all the water is gone. You will be left with the sugar, and then you can weigh it. First weigh your pot before pouring the soda in. Now boil the soda on the stovetop. When the water evaporates, you'll be left with a syrupy sugar. A can of soda has 39 grams of sugar in it. That equates to about 7 1/2 teaspoons. Fruit juice conta...
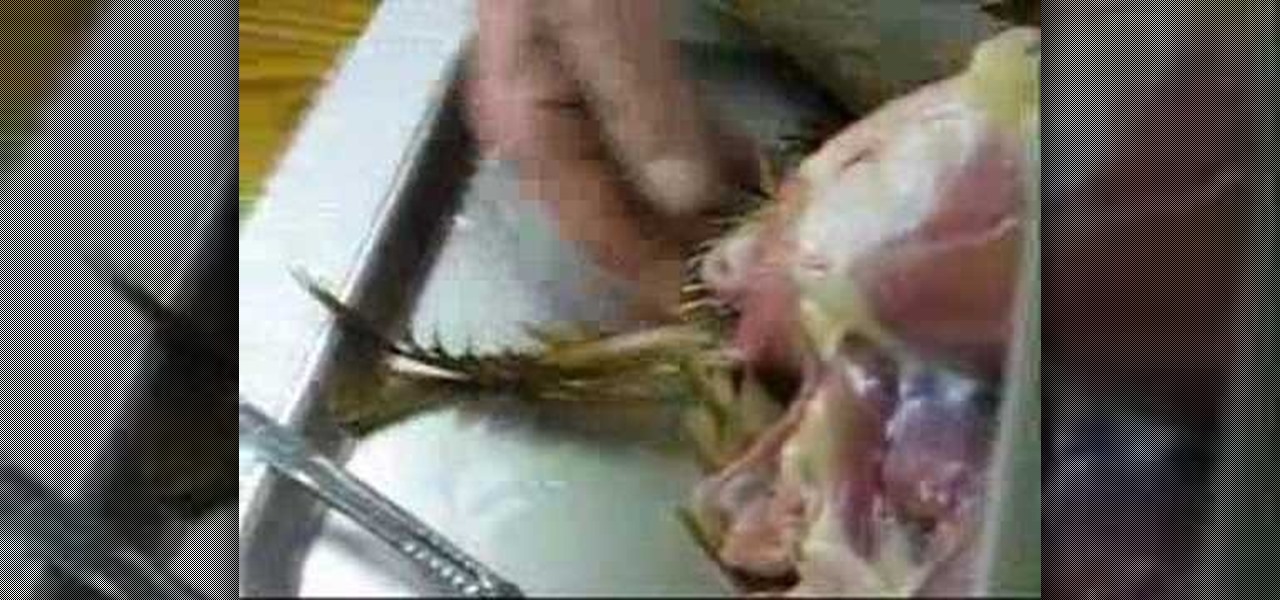
How To: Dissect a chicken for an anatomical look
Failed out of veterinarian school? No problem, just watch this video tutorial on dissecting a chicken (avian) to get you back on your feet. This demonstration and lecture of is by an eminent anatomist that will show you how to do it correctly.

How To: Make solid rocket fuel ignition with zinc and sulfur
Once used as solid rocket fuel because the reaction requires no oxygen, sulfur and zinc react vigorously. The reaction with zinc produces flame and a near explosion. Sparks fly and smoke billows in this dramatic chemical demonstration.

How To: Make your own nylon
In this tutorial, we learn how to make your own nylon. You will need: pipettes, pipette filler, forceps, beaker, stir rod, sebacoyl chloride and hexanediame solution. Now, pour some of the hexanediame solution into the small beaker. Add in a food coloring if you want to make this a specific color. After this, add in 4 cc's of sebacoyl chloride and carefully drip into the side of the beaker. You should see a layer of where the two liquids are after this. Now, take your tweezers and reach into ...

How To: Make boric acid from borax
This is a video tutorial in the Education category where you are going to learn how to make boric acid from borax. For this experiment you will need borax (disodium tetra borate) and conc. hydrochloric acid. Take 25 ml of hydrochloric acid and dilute it with 75 ml of water. Next take 6 - 7 gms of borax and dissolve it in boiling water. Now add equal amount of hydrochloric acid. Crystals of boric acid will start forming. They are completely insoluble in cold water. After about half an hour, fi...