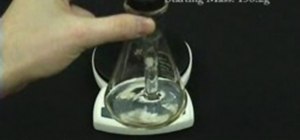
Try out this science experiment... watch this video tutorial to learn how to illustrate the scientific concept of conservation of mass. This is purely educational, and not as exciting as some other demonstrations, but this does clearly illustrate the concept of conservation of mass.
When compounds react, no atoms are created or destroyed. This means that the end product must weigh the same as the initial reactants.
Here, lead nitrate and potassium iodide are both placed inside of a stoppered flask. They are kept separate as one was placed inside of a test tube. When the flask is inverted, a double replacement reaction takes place. Since the stopper prevents anything from escaping, the end mass is still the same.
This is the reason that chemical equations need to be balanced. There are no new atoms being formed.
The yellow product is insoluble lead iodide.
This is an easy science experiment you can do right at home.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.






















Be the First to Comment
Share Your Thoughts