In this experiment, we're going to get Mn2O3 (manganese(III) oxide) from MnO2 (manganese(IV) dioxide). Mn2O3 forms brightly red or a dark red colored crystal. It is used in Li-ion batteries, since (in a form of a crystal) it conducts electricity (much like MnO2).
There isn't really much to say about this compound; it's practically useless, although MnO2 has wide use.
Step 1: Ingredients & Tools
For this experiment, we're going to need 200g of MnO2

Any kind of a blowtorch.

Something to put the MnO2 in (it needs to withstand heat). Over here, I used a copper plate.

A gas mask (optional).
Step 2: Preparing
Place the MnO2 into your plate
Step 3: Synthesis
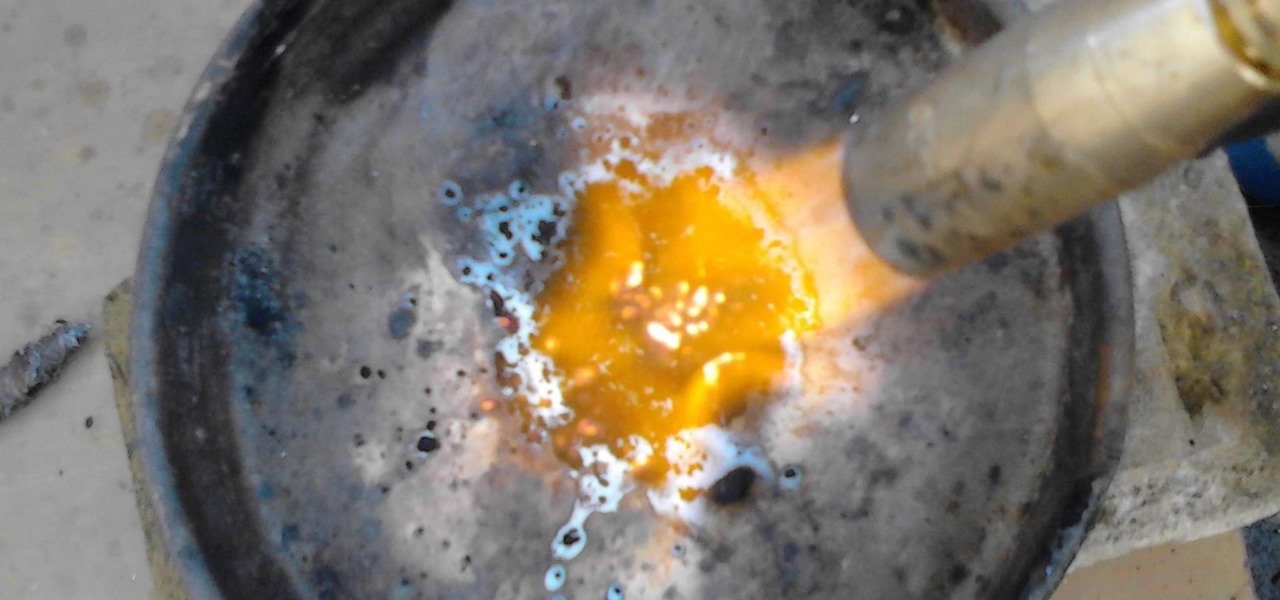
ATTENTION: If you are easily grossed out, put on a gas mask. MnO2 smells foul when burning. Turn on your blowtorch and apply the flame to MnO2 for about 45 seconds.

After you have applied the flame for 45 seconds, let the compound cool down. Even now you'll see red colored pieces of Mn2O3

Repeat the process until the content in the plate has turned completely red.
ATTENTION 2: When most of the MnO2 has turned to Mn2O3, the smell will become horrid (it'll smell of vomit and gunpowder), so I really advise you at least put your shirt over your nose.
Have fun.
Just updated your iPhone to iOS 18? You'll find a ton of hot new features for some of your most-used Apple apps. Dive in and see for yourself:

























Be the First to Comment
Share Your Thoughts