Science Experiments How-Tos


How To: Calculate weighted atomic mass
This video is about Level 2 atomic concepts, specifically the Pennium Lab. This helps you understand how atomic masses are derived for the periodic table. This teacher demonstrates how to do the calculation. He first reviews the Isotopes as they are different masses of the same type of atoms. He uses chromium as his example. It has a different number of neutrons in the nucleus. Using the equation, it could have either 26, 28, 29 or 30, with its 24 protons. So he subtracts the atomic number fr...

How To: Measure pH levels with paper and meters
If you want to know the best way to get an accurate pH measurement, this shows the lab equipment needed and the processes used. When doing chemical reactions, sometimes the acidity or the basicity is important. This is usually defined as pH and measuring it can be very useful for getting the reaction right. There are a few ways to measure pH, and the simplest, cheapest, most reliable method is paper. But that's not all. See a whole lot of ways in this two-part video.

How To: Calculate friction in a body without acceleration
This video shows the viewer how to calculate friction in a body that it is not accelerating. The simple answer is that the force of friction will be equal to the force needed to maintain the constant speed. This means that is you pull a wooden block along a carpet at a constant speed and a Newton meter between you and the block measures 5 Newton’s then the force of friction between the carpet and the block is exactly 5 Newton’s. If the object is accelerating then this rule does not apply.For ...
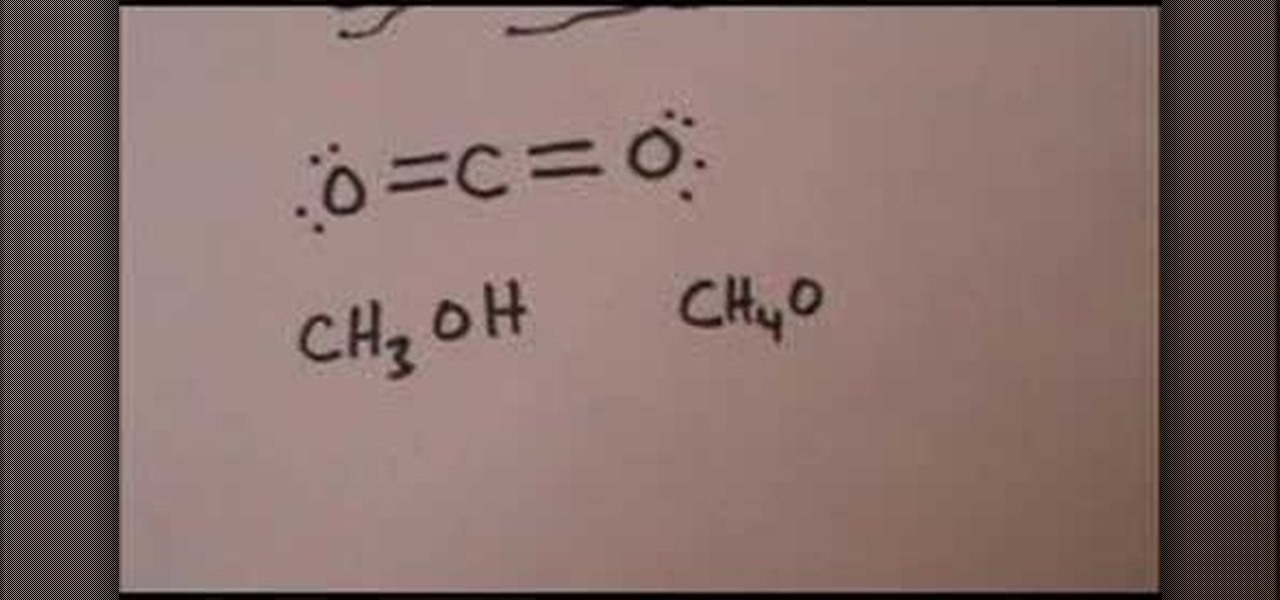
How To: Draw the Lewis structure for carbon dioxide & methanol
In this Education video tutorial you are going to learn how to draw the Lewis structure for carbon dioxide & methanol. Carbon dioxide is CO2. Oxygen atoms don’t bond together. So, carbon atom has to be in the center. Oxygen has 6 valence electrons and carbon has 4. Hence, carbon wants to form 4 bonds and each oxygen atom wants to form 2 bonds. So, two each electrons of carbon will bond with 2 electrons of each oxygen atom. The Lewis structure for CO2 will be ( …. O=C=O….). The 4 dots within t...

How To: Make a baking soda volcano project
In this video, we learn how to make a homemade volcano. You will need: 6 cups of all purpose flour, 2 cups of salt, 4 tablespoons of cooking oil, an empty plastic soda bottle, dish washing liquid, food coloring (optional), vinegar, 2 tablespoons of baking soda, a cooking pan, a mixing bowl and spoon, 2 cups of warm water, plastic container. First, add 6 cups of water to large mixing bowl. Now, add 2 cups of salt, 2 cups of water and 4 tablespoons of cooking oil. Mix this together with your sp...

How To: Draw the Lewis dot structures for 2 types of alcohol
In this video, you will learn how to draw the Lewis Dot structure for CH3CH2OH, or drinking alcohol. To draw the first type of alcohol, draw H, C, C, O, and H horizontally. Now draw, an H above and below both C. Draw the dot structures in between each letter. Hydrogen has one valance electrons, Carbon has four, and Oxygen has six. Now, connect the Lewis Dot Structures. If you are given C2H6O, write down C, O, and C horizontally. Draw a line between the O and the C's. The Carbons will have thr...

How To: Build a simple barometer with household materials
ARHSphysics shows viewers how they can build a simple barometer using household items! First, you need to get a container that is the height of an index card. Your best bet is to get an empty soup can of food can. Make sure you wash the can. Now, you will need a latex glove, cut a semi circle from the glove and attach this to the top of the can using a rubber band. Get two straws and attach them together at the ends. Cut a slit in each one and attach the straws. Attach the straw to the top of...

How To: Make a candle wax explosion
In this video, you'll see how and why wax can be a deadly. This is how it works,When the test tube is submerged in cold water, the glass forms tiny cracks. The Water enters gets into the tube and vaporizes causing a micro explosion. The hot wax is then ejected from the tube quickly as oxygen is displaced from the tube itself. Be careful, and make sure to be as safe as possible when trying this experiment at home.
How To: Convert American Pounds to Kilograms
In this video, learn how to convert the American pound (lb.), a United States customary unit of mass, into kilograms (kg), a metric unit of mass in the International System of Units (SI).

How To: Test for alkaloids with Pepto-Bismol tablet
This video shows you an alternative procedure to bismuth subnitrate, using Pepto-Bismol tablets. You'll learn how to test for alkaloids, similar to a toxicology report. Put on your goggles, for this great home science experiment.
How To: Draw atoms of different elements
An atom is a basic unit of matter consisting of a dense, central nucleus surrounded by a cloud of negatively charged ions. The nucleus itself is a mixture of positively charged protons amd electrically neutral neutrons. Different groups of elements have respective atomic numbers. You can use the periodic table as a tool to draw atoms of elements. The periodic table is organized into periods, groups and families. This video is a tutorial that reviews the subatomic particles found in an atom. I...

How To: Make dancing drops
This video shows you how to make your liquid dance. Its happens like so, at around 300 degrees Fahrenheit a phenomenon in which a liquid, at close contact with a mass significantly hotter than the liquid's boiling point, rides upon an insulating vapor layer which keeps that liquid from boiling and evaporating. What is this strange phenomenon? The "Leidenfrost effect" of course, and with Mr. G at the helm it's also a lot of fun.

How To: Make faux night vision goggles at home
Ever wonder how to make your very own faux night vision goggles? Well, Mr. G shows you exactly how to make your own night vision "glasses". Step by step, soley using household products. This experiment is just too good to be true. A foolishly easy experiment that will make you laugh and cry at the same time. This video is just for fun, don't let it fool you!

How To: Do a simple experiment using copper wire and battery
In this video Mr.G puts a new spin on magnets and bare copper wire with just a simple battery. Motion via magic? Not quite, but pretty darn close! Join Mr. G, and build your own motor with its own unique new spin. This is a fun, easy, do it at home experiment.

How To: Reduce Static Electricity
Static electricity might seem like magic but you don't have to be a wizard to get rid of it. There are simple steps you can take to reduce the amount of static electricity in the air or on you by using some items you probably have at home.

How To: Make hot ice using Sodium Acetate Trihydrate
This video in the Education category will show you how to make hot ice using Sodium Acetate Trihydrate. For this purpose you will need a pan, 100g of Sodium Acetate Trihydrate, 25ml of water, a wooden spoon and a glass. Take the Sodium Acetate Trihydrate and put 100g in the pan. Then place the pan on a stove and turn to medium heat. The Sodium Acetate Trihydrate will start to melt. In about five minutes when it has melted fully, add 25ml of water. Let the solution simmer for two minutes while...

How To: Make an extra long ping pong smoke bomb
The video shows how to make an extra long ping pong smoke bomb. For this we need a long sheet of aluminum foil (length of the foil depends on the number of ping pong balls we use), 12 ping pong balls is used in the video (any number of balls can be used), a pair of scissors and a lighter.

How To: Balance chemical equations properly
Confused by the equations in chemistry class? This tutorial is here to help! Clark College Tutoring and Writing Center tutors Kevin Martin and Joey Smokey explain how to balance chemical equations, providing examples and tips in this two part video series. You will definitely improve your test scores after watching this step by step program.

How To: Explode soda bottles with dry ice & make a spring
In this video Dave Spencer shows you how to make soda bottles explode using dry ice. You will need dry ice pellets plastic soda pop bottles , and gloves (dry ice can be held in your hands but should be kept moving and not held up too long as it can cause severe frost bite). The presenter asks you to note that this activity is illegal in the state of Utah. Crushed dry ice is inserted into the soda pop bottle. The soda pop bottle is then shaken up vigorously and placed into the ground. You shou...

How To: Build a DIY electromagnet for cheap
This video shows the viewer how to make an electromagnet using common items. This is done using a broken microwave, a spool, a cordless drill, a battery and a coat hanger. The microwave transformer is dismantled and the enamel insulated wire is removed. Using the cordless drill the wire is then coiled around the spool. The iron coat hanger should then be cut into small pieces and placed in the middle of the spool. It should be secured tightly. The battery was then connected to the wired coile...

How To: Make "Hot Ice" with Sodium Acetate Crystals
If you've ever used a heating pad or hand warmer, you essentially know what "hot ice" is. It's supersaturated sodium acetate, and it's actually fairly easy to make at home out of sodium acetate crystals. You can also make it out of vinegar and baking soda (directions at the bottom of this article).

How To: Turn water into ice without a freezer
Here's a fun experiment you can do that will demonstrate the effects that pressure has on the freezing point of a liquid. You will amaze your friends as you do what seems to be impossible, turning water into ice without sticking it in the freezer.

How To: Calculate and understand the concept of molar mass
Two college students; Kevin Martin and Joey Smokey introduce the concept of Molar Mass. They start of by explaining what molar mass is, which is the relationship of a mole and a gram, it totals up the weight(in g)of a molecule. An example: say you have this compound, Sodium phosphate (Na3PO4). You know you have three sodium atoms, one phosphorus atom, and four oxygen atoms. You basically find the weight of each atom, if you have three sodium atoms, you multiply it's atomic mass by 3 (the numb...

How To: Make a cloud, then make it disappear
This video features a really cool science experiment that is easy to do and fun to watch. Items you will need are a plastic 2-liter bottle with a sports bottle type cap (the kind you pull up on in order to sip liquids through the top), about a quarter of a cup of water and two matches. First, take the cap off the bottle and pour the water into the bottle before putting the cap back on the bottle. Then, simply open the pull top on the cap (so that when the bottle is squeezed and released air i...

How To: Make hot ice from scratch
To make instant hot ice or sodium acetate, first pour two jugs of vinegar minus one cup into a large pot. Then, slowly add baking soda to the mixture and stir it. When the vinegar and baking soda no longer react, stop adding baking soda. Next, add the rest of the vinegar to the pot. Boil half of the solution off and cool it down to room temperature. For better filtering, add charcoal to the mixture. Filter out the charcoal with a wire sifter. Heat up the solution again and filter it through c...

How To: Make "hot ice"
In this video tutorial, viewers learn how to make "hot ice". Users will need sodium acetate. Begin by putting the sodium acetate into a pan. Add a small amount water to the sodium acetate. Heat the mixture on a stove until the sodium acetate has dissolved. Pour the solution into a container. Do not pour in any undissolved crystals. Put the container into the freezer or refrigerator for a while. When the solution cools down to room temperature, take it out. Touch the sodium acetate and it will...

How To: Balance chemical equations with MyTutorBuddy
Learn how to balance chemical equations with MyTutorBuddy. Learn about this in this video tutorial. There are four easy steps to do this. Step #1 – place 1 by the most complex compound. Step #2 – balance anything that is not an element. Step #3 – balance the elements. Step #4 – multiply by the lowest common multiple. The 4th step doesn’t always come in to play. The video demonstrates with an equation: C3H8 + O2 -> H2O + CO2. But, this equation is not balanced. Using the 1st three steps, the v...

How To: Make a simple ping pong smoke bomb with one ball
This video demonstrates the easiest and fastest smoke bomb that a person can make. Supplies include aluminum foil, standard ping pong ball, a pen or pencil, and a flame lighter. Wrap the ping pong ball with foil, being extra careful not to tear the aluminum foil. Use the pen to create a funnel shape with the foil. Remove the pen. Now, light the bottom while holding the top. The smoke is toxic, so don't inhale. Make sure the smoke bomb is on a non-flammable surface as it gets very hot. Also, w...

How To: Calculate molecular formula using molar mass
The man was requested to solve a short, basic chemistry exercise. He starts by explaining the meaning of the terms involved in the problem: molecular mass, empirical formula and molecular formula. He also explains the way the are connected, for a better understanding of the way the exercise is solved.

How To: Make hydrogen gas & an explosion
First you need to make hydrogen gas which require a few material. Such as a glass bottle, a Full table spoon of aluminum (beer or soda can will work), a half a cup of cold water, a table spoon or two of caustic soda, a funnel, a medium size container of water, a way of cutting the aluminum, one or two balloon's, and some safety glasses just incase something goes wrong.

How To: Make an awesome colored smoke bomb
The video describes an easy at home process for making smoke bombs. The items you will need are as follows:

How To: Balance chemical equations with Olivia and Andrea
Olivia and Andrea created a song to the tune of "I'll stand by you" by the Pretenders to teach you how to balance chemical equations. A chemical equation requires coefficients in order to be balanced. You can balance a chemical equation by making use of the Periodic table. The example reaction in the song is that of aluminum and oxygen to produce aluminum oxide (Al + 02 -> Al203). You can systematically add coefficients to the reactants and products to balance the equation. A chemical equatio...

How To: Understand organic molecules & oxidation in Chemistry
Do you understand organic molecules or oxidization in chemistry? If you answered no then this is the video you need to watch. This video will show you grade 12 chemistry, with organic models, and will teach you about oxidization. In less than 4 minutes you will have a much better understanding of the topic. This is demonstrated with a few common chemicals which include potassium dichromate and ascorbic acid. Ascorbic acid or vitamin C is found in many foods that we eat including fruits and ve...

How To: Make neat "hot ice"
In this video tutorial, viewers learn how to make "hot ice". Begin by adding water into a pan and heat it until it’s simmering, but not boiling. Add the sodium acetate to the water. Keep adding the sodium acetate until the water cannot dissolve it anymore. Stir constantly. Now pour the solution into a glass or container. Do not pour in any undissolved crystals. Place the solution into the refrigerator for 45 minutes. Now pour the solution into a container. The liquid will instantly turn into ...

HowTo: Reboot Your Corpse
IEEE Spectrum examines the practice of cryonically freezing the dead, with the intent to "reboot" when medical advances are prepared to undo their death.

How To: Understand organic molecules & elimination reaction
This video helps us understand the organic molecules and elimination reaction. Take some sugar in a beaker. Sugar has 12 carbon atoms, 22 hydrogen atoms and 11 atoms of oxygen. The sulphuric acid is poured into the sugar and the color change is observed. The color of the sugar gradually changes into black. The sulphuric acid causes an exothermic reaction which releases a large amount of sulphur dioxide gas. All the water (containing hydrogen and oxygen atoms) is eliminated out of the sugar du...

How To: Make manganese dioxide electrodes
Various electrochemical reactions requires that anodes do not degrade when used. Carbon is cheap, but degrades easily and platinum is extremely expensive. In a previous video, you learned "How to make cobalt and manganese nitrates", and you saw that titanium could be used as a cathode, but not as an anode due to an effect called passivation.

How To: Convert Celsius to Fahrenheit
Can't remember how to convert Celsuis temperatures to Farenheit? If your old science lessons are escaping you, then your answer is here. Use the simple formula outlined in the steps in this video to make the proper conversion every time.

How To: Make cobalt and manganese nitrates
In order to make manganese dioxide electrodes, you're going to need cobalt nitrate and manganese nitrate to do it. Making cobalt nitrate is fairly easy, but making the manganese nitrate is a little more complicated. But not impossible.
How To: Find molar mass
It’s time for science. You are able to calculate the molar mass for a compound using the periodic table and the amount of compound involved. You might need to know this in your everyday life but you will definitely need this in a chemistry class.






